**Please note this article is part of a larger series that was first released in the April 2021 Monthly Playbook. If you would like to receive the complete monthly playbook you can sign up here
Part 5: Cannabis Extraction Methods
The separation of the wide array of compounds in Cannabis sativa is becoming more important as regulations on cannabinoid-containing products become more stringent. There are a few common methodologies used for extraction in the cannabis industry.
Depending on the intended final product, incoming processing can include separation of the flower portions with the highest concentration of cannabinoids and terpenes. Then the extraction of the terpenes and the cannabinoids can begin. Though mechanical separation methods exist, this article will focus on the most common solvent-based
extractions: hydrocarbon, ethanol and super-critical CO2. Solvent extraction methodologies have been around for millennia since the ancient Egyptians made tinctures by soaking herbs in ethanol. Tinctures are created by putting the herbs into a liquid, usually alcohol, to extract the flavors, healing components, etc.

The basic solvent extraction is based on historical methods, although we have made technological advances to help us control the processes over the centuries. Today, common organic solvents include ethanol [1], butane, and propane.[2] Water extraction will not be covered in this article due to its low yields of cannabinoids and terpenes.
Alcohol extraction uses ethanol and is one of the most common and efficient methods of extracting the cannabinoids. The initial step is to soak the raw material in ethanol to remove the trichomes. The polar end (-OH group) of the molecule helps dissolve the hydrophilic compounds, such as chlorophyll. The non-polar end (C2¬H5) helps to
dissolve the hydrophobic compounds such as the plant waxes, oils, cannabinoids, and terpenes.
The process can be done in either warm or cold ethanol. In warm or room temperature ethanol, the cannabinoids dissolve quickly leading to a high yield of cannabinoids. However, the warm ethanol extraction process also dissolves plant lipids including the chlorophyll, which causes a strong bitter taste. By dropping the temperature of the ethanol to less than -30˚C (-22˚F), you decrease the solubility of all the compounds leading to a much slower dissolution of the products into the ethanol. However, it is also below the freezing point of many plant waxes which means that many of the compounds will be solids. The impurities separate from the cannabinoids and can be easily removed.
Hydrocarbon extraction generally uses either propane or butane. Propane and butane are small hydrocarbons, made only of carbon and hydrogen atoms, that are non-polar with low boiling points, (-44˚F and 32˚F, respectively) [3]. The initial extraction washes the raw material with the cold hydrocarbon.
The non-polarity of the molecules helps easily dissolve the cannabinoids, waxes, fats and lipids. Unlike ethanol, there is no polar end to help with the dissolution of certain undesired compounds such as chlorophyll. The terpenes are also easily dissolved in the hydrocarbons, though the flavonoids have limited solubility. The hydrocarbons with the products of interest can be separated by flowing the mixture into a separate area and raising the temperature.[4] With low boiling points, the hydrocarbons evaporate at -44˚F (Propane) and 32˚F (Butane) leaving behind the waxes, fats, lipids, cannabinoids, and terpenes.
After the separation of the hydrocarbons from the extract, the propane or butane can be recirculated though the biomass creating a closed-looped system. The result is an extract that is relatively free of inactive plant matter such as chlorophyll. Hydrocarbon extraction is losing popularity primarily due to regulations for handling propane/butane
and stigma attached to a using a hazardous chemical for the extraction.
The final extraction method uses super-critical CO2. Outside the cannabinoid industry, supercritical extraction methods are used for the production of high-quality hempseed oil, extracting caffeine from coffee, removing pesticides from agriproducts, etc. [5] At standard temperatures and pressures (room temperature and sea-level atmospheric
pressure), all molecules are in their natural state of matter: solid, liquid, gas, or plasma. One can change the state of matter by changing the temperature or the pressure or both. A good example of this would be creating ice cubes in your freezer. Without changing the pressure, you can turn the water into ice by lowering the temperature.
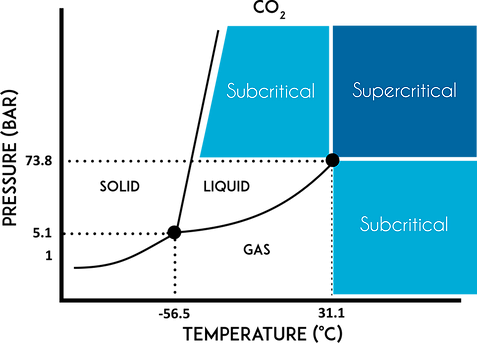
When modulating the temperature and pressure of a system, liquids and gases can hit a critical point where they exhibit characteristics of both liquid and gas. They take up the entire space (more compressible) like gases and have liquid-like densities. This is called a supercritical fluid.[7] Figure 2 shows the phase diagram of Carbon Dioxide (CO2).
Using CO2 has several advantages, it is nonflammable, non-toxic, relatively inert, abundant and inexpensive. [8, 9]
The other main advantage is that at a temperature of 31˚C, you can maintain the supercritical liquid at 74 bar.[6, 7] The various components of cannabis have different solubilities at different temperatures and pressures, thus allowing a clean extraction of the target compounds. However, one study has shown that the concentrations of different products can be extracted at different rates, so the extract should be analyzed. [2, 8, 10]
References:
- J. Plotka-Wasylka, M. Rutkowska, K. Owczwarek, M. Tobiszewski and J. Namiensnik, “Extraction with environmentally friendly solvents,” Trends in Analytical Chemistry, vol. 91, pp. 12-25, 2017.
- M. May, “The Best Extraction Methods for Marijuan concentrates,” 3 May 2018. [Online]. Available: https://www.analyticalcannabis.com/articles/the-best-cannabis-extraction-methods-for-marijuana-concentrates-300434.
- L. G. Wade, Organic Chemistry, 4th Edition, NJ: Prentice hall, 1999.
- Marijuana Business Magazine, “Choosing the right cannabis extraction method: Experts weigh in on CO2, hydrocarbon & ethanol,” Marijuana Business Magazine, 2018.
- Le Portail Des Fluides Supercritiques, “Applications,” Le Portail Des Fluides Supercritiques, [Online]. Available: http://www.supercriticalfluid.org/Applications.149.0.html.
- M. Ollero and D. Touboul, “Lipidomics by Suprictical Fluid Chromotography,” International Journal of Molecular Science, vol. 16, no. 6, 2015.
- P. Atkins and J. de Paula, Physical Chemistry, 7th Edition, Oxford Univerisity Press, 2002.
- C. L. Ramirez, M. A. Fanovich and M. S. Churio, “Cannabinoids: Extraction, Methods, Analysis, and Physiochemical characterization,” Studies in Natural Products Chemistry, vol. 61, pp. 143-173, 2019.
- Airgas, “Safety Data Sheet – Carbon Dioxide,” 12 February 2018. [Online]. Available: https://www.airgas.com/msds/001013.pdf.
- A. Beadle, “Advances in Cannabis Extraction Techniques,” 25 June 2019. [Online]. Available:
https://www.analyticalcannabis.com/articles/advances-in-cannabis-extraction-techniques-311772.


