Jeffrey N. Keller PhD, Rapid Analytics E: [email protected]
The use of cannabis and cannabis-derived molecules for medicinal purposes has occurred for Millennia. The incorporation of cannabis into Western medicine has occurred relatively recently as the result of the identification of cannabinoid receptors and the elucidation of the endocannabinoid system.1-3 An ever-expanding list of bioactive molecules derived from cannabis has been established including both major and minor cannabinoids, terpenes, and flavonoids.4,5 Ultimately these scientific efforts resulted in Food and Drug Administration (FDA) approval for the use of synthetic cannabinoids for several medical conditions,6 and the approval of cannabis-derived cannabidiol (CBD) for select forms of epilepsy.7
Despite the considerable advances made in the research and development (R/D) of cannabis-based therapies, the reality is that our understanding of the therapeutic potential of cannabis-based molecules lags far behind what is known for many other botanicals.8,9 Longstanding impediments to the R/D of cannabis-based therapies include:
- Legacy of Federal agency roadblocks
- Lack of clarity on Federal agency roles/responsibilities
- Lack of guidance on the legality of physician-patient discussions of medical cannabis use
- Lack of R/D infrastructure
- Lack of rigor in regard to the quality/amount of cannabinoids in commercially available products
- Lack of education on the need and utility of research
- Fragmentation of the cannabis industry
In December of 2022, the president of the United States signed into law the medical marijuana and cannabidiol research expansion act (MMCREA).10 The MMCREA directly addresses several of the obstacles that have slowed the pace of cannabis research. In particular, the MMCREA removes and streamlines several longstanding regulatory hurdles to cannabis-related research and provides support for physician-patient conversations on the topic of medical cannabis. In addition, the MMCREA requires federal agencies to report annually to Congress on the efforts that have been taken to ensure an adequate supply of research-grade cannabis and to report on the latest health benefits/risks of medical cannabis.
Taken together, the MMCREA unequivocally improves multiple aspects of the research environment surrounding medical cannabis R/D (Figure 1). However, it is important to point out that several key obstacles to cannabis-related R/D remain largely intact and unaffected by the MMCREA (Figure 1). Moving to a state of robust cannabis-related R/D will require addressing the fragmentation, lack of infrastructure, and lack of education that currently impede the creation of a robust cannabis R/D environment.
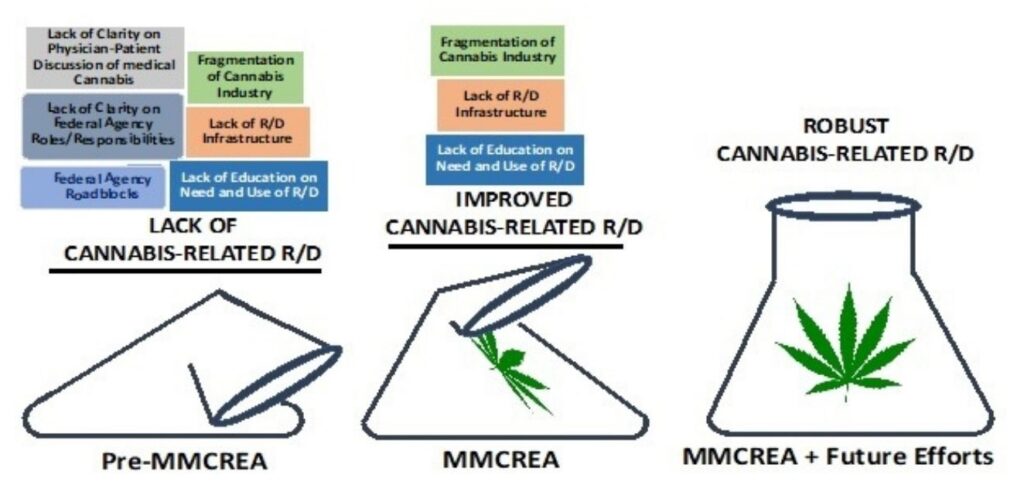
Figure 1. The medical marijuana and cannabidiol research expansion act (MMCREA) improves many, but not the majority, of obstacles that have impeded cannabis-related R/D.
To be continued, next month.
Part 2: How to Leverage the Success of MMCREA Moving Forward
Editors’ Note: This is an excerpt from our Monthly Playbook. If you would like to read the full monthly playbook and join the thousands of others you can sign up below.


